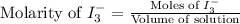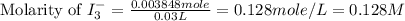Chemistry, 25.07.2019 22:20 nataluarenhg6924
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a known concentration of s2o2−3(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s2o2−3(aq)+i3(aq)⟶s4o2−6(aq)+3i−(a q) given that it requires 29.6 ml of 0.260 m na2s2o3(aq) to titrate a 30.0 ml sample of i−3(aq), calculate the molarity of i−3(aq) in the solution.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
The amount of i−3(aq) in a solution can be determined by titration with a solution containing a know...
Questions

Mathematics, 15.11.2019 07:31

History, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31


English, 15.11.2019 07:31

Physics, 15.11.2019 07:31

Chemistry, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31




Chemistry, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31


Physics, 15.11.2019 07:31

History, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31

History, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31

Mathematics, 15.11.2019 07:31

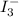 in the solution is, 0.128 M
in the solution is, 0.128 M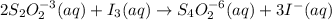
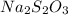 .
.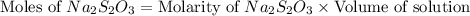
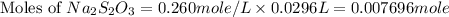
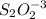 react with 1 mole of
react with 1 mole of 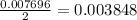 mole of
mole of