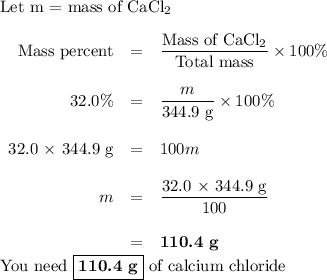
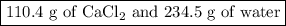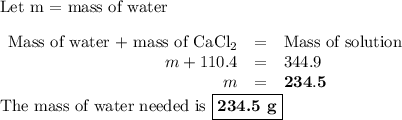How many grams of cacl2
are needed to make 344.9
g of a solution that is 32.0
% (m...

Chemistry, 25.07.2019 23:10 mickimlvn6128
How many grams of cacl2
are needed to make 344.9
g of a solution that is 32.0
% (m/m) cacl2
in water? note that mass is not technically the same thing as weight, but % (m/m) has the same meaning as % (w/w).
grams cacl2=
g
how many grams of water are needed to make this solution?
grams h2o=

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
Questions

History, 29.10.2020 18:30




Biology, 29.10.2020 18:30

Chemistry, 29.10.2020 18:30

History, 29.10.2020 18:30



Mathematics, 29.10.2020 18:30

English, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30

Geography, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30


Business, 29.10.2020 18:30

English, 29.10.2020 18:30