

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
For the reaction below, kp 5 1.16 at 800.8c. caco3(s) 34 cao(s) 1 co2(g) if a 20.0-g sample of caco3...
Questions

History, 17.12.2020 23:00



Mathematics, 17.12.2020 23:00

Mathematics, 17.12.2020 23:00




History, 17.12.2020 23:00

Mathematics, 17.12.2020 23:00


Mathematics, 17.12.2020 23:00



Mathematics, 17.12.2020 23:00

Spanish, 17.12.2020 23:00




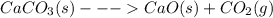
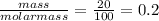
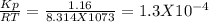
![\frac{[CO_{2}][CaO]}{[CaCO_{3}]}= \frac{x^{2} }{(0.2-x)}=1.3X10^{-4}](/tpl/images/0133/4658/99790.png)
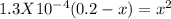
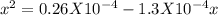
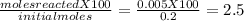 %
%


