
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to nitric oxide by the high-temperature reaction 4 nh31g2 + 5 o21g2 ¡ 4 no1g2 + 6 h2o1g2 (a) how is the rate of consumption of o2 related to the rate of consumption of nh3? (b) how are the rates of formation of no and h2o related to the rate of consumption of nh3

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
In the first step of the ostwald process for the synthesis of nitric acid, ammonia is converted to n...
Questions



Mathematics, 04.11.2021 21:00

Advanced Placement (AP), 04.11.2021 21:00





Geography, 04.11.2021 21:00

Mathematics, 04.11.2021 21:00

Mathematics, 04.11.2021 21:00



Mathematics, 04.11.2021 21:00


Mathematics, 04.11.2021 21:00


Mathematics, 04.11.2021 21:00


Social Studies, 04.11.2021 21:00

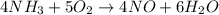
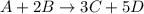
![rate=-\frac{\Delta [A]}{\Delta t}=-\frac{1}{2}\frac{\Delta [B]}{\Delta t}=\frac{1}{3}\frac{\Delta [C]}{\Delta t}=\frac{1}{5}\frac{\Delta [D]}{\Delta t}](/tpl/images/0133/4682/3644a.png)
![rate=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}=-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}](/tpl/images/0133/4682/51a20.png)
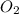 related to rate of consumption of
related to rate of consumption of 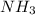 as:
as:![-\frac{1}{5}\frac{\Delta [O_2]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/1a857.png)
![\frac{\Delta [O_2]}{\Delta t}=\frac{5}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/ce2db.png)
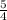 the rate of consumption of ammonia.
the rate of consumption of ammonia.![\frac{1}{4}\frac{\Delta [NO]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/91a55.png)
![\frac{\Delta [NO]}{\Delta t}=-\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/c5068.png)
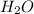 to the rate of consumption of ammonia would be:
to the rate of consumption of ammonia would be:![\frac{1}{6}\frac{\Delta [H_2O]}{\Delta t}=-\frac{1}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/41d66.png)
![\frac{\Delta [H_2O]}{\Delta t}=-\frac{6}{4}\frac{\Delta [NH_3]}{\Delta t}](/tpl/images/0133/4682/66644.png)
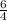 times that is 1.5 times to the rate of consumption of ammonia.
times that is 1.5 times to the rate of consumption of ammonia. 

