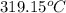Chemistry, 26.07.2019 04:10 hairyears3394
What celsius temperature, t2, is required to change the volume of the gas sample in part a (t1 = 23 ∘c , v1= 1.69×103 l ) to a volume of 3.38×103 l ? assume no change in pressure or the amount of gas in the balloon.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
What celsius temperature, t2, is required to change the volume of the gas sample in part a (t1 = 23...
Questions





English, 20.05.2020 19:58




History, 20.05.2020 19:58

Mathematics, 20.05.2020 19:58


Physics, 20.05.2020 19:58

Mathematics, 20.05.2020 19:58

Mathematics, 20.05.2020 19:58

Social Studies, 20.05.2020 19:58

Physics, 20.05.2020 19:58




English, 20.05.2020 19:58

![\frac{T_{2} }{V_{2} } = \frac{T_{1} }{V_{1} } \\Which can be rewritten as T_{2} = \frac{T_{1} V_{2} }{V_{1} }if you make T2 the subject of the formula. This formula is true only if temperature is in Kelvin not degrees Celsius so T1 must be converted to KelvinNow to calculate T2[tex]T_{2}= \frac{296.15K*3.38.10^{3}L }{1.69.10^{3}L }= 592.3K](/tpl/images/0133/6170/71b6f.png) =
=