
Chemistry, 26.07.2019 20:30 joeblaszak4776
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heated at a constant pressure of 0.5 bar from 100 °c to such a temperature that the specific volume increases by 2.5 times. if the amount of heat that must be added to accomplish this change is 500 kj, calculate the final temperature of the steam, the expansion work, and the change in internal energy.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Three kilograms of steam is contained in a horizontal, frictionless piston and the cylinder is heate...
Questions



Mathematics, 20.06.2020 20:57


Mathematics, 20.06.2020 20:57




Biology, 20.06.2020 20:57

Social Studies, 20.06.2020 20:57









Mathematics, 20.06.2020 20:57

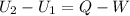
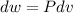
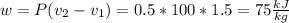 (There is a multiplication by 100 due to the conversion of bar to kPa)
(There is a multiplication by 100 due to the conversion of bar to kPa)
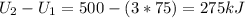
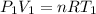
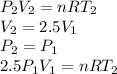
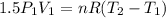
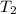 :
: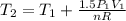
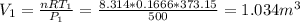
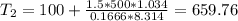 ºC
ºC

