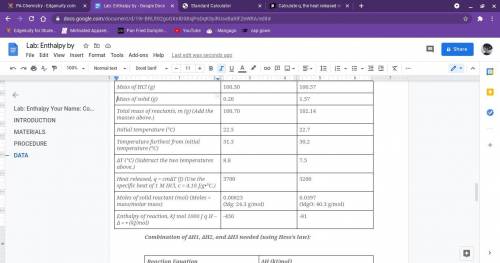
Calculate q, the heat released in each reaction.
use the equation q = cmåt.
(use c = 4.1...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

You know the right answer?
Questions


History, 09.11.2020 15:30

Mathematics, 09.11.2020 15:30



English, 09.11.2020 15:30



Mathematics, 09.11.2020 15:30

Business, 09.11.2020 15:40

English, 09.11.2020 15:40

Mathematics, 09.11.2020 15:40



English, 09.11.2020 15:40

World Languages, 09.11.2020 15:40

Mathematics, 09.11.2020 15:40

Mathematics, 09.11.2020 15:40

Mathematics, 09.11.2020 15:40

Mathematics, 09.11.2020 15:40