
The equilibrium constant, kc, for the following reaction is 77.5 at 600 k. co(g) + cl2(g) cocl2(g) calculate the equilibrium concentrations of reactant and products when 0.442 moles of co and 0.442 moles of cl2 are introduced into a 1.00 l vessel at 600 k. [co] = m [cl2] = m [cocl2] = m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
The equilibrium constant, kc, for the following reaction is 77.5 at 600 k. co(g) + cl2(g) cocl2(g) c...
Questions


Biology, 24.01.2020 06:31

Mathematics, 24.01.2020 06:31



English, 24.01.2020 06:31


History, 24.01.2020 06:31

History, 24.01.2020 06:31


Mathematics, 24.01.2020 06:31




Business, 24.01.2020 06:31


Physics, 24.01.2020 06:31

Biology, 24.01.2020 06:31

History, 24.01.2020 06:31

Mathematics, 24.01.2020 06:31

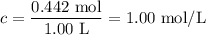
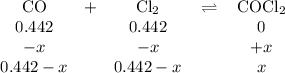
![K_{\text{c}} = \dfrac{\text{[COCl$_{2}$]}}{\text{[CO][Cl$_2$]}} = \dfrac{x}{(0.442 - x)^{2}} = 77.5\\\begin{array}{rcl}\\\dfrac{x}{(0.442 - x)^{2}} & = & 77.5\\\\x & = & 77.5(0.442 - x)^{2}\\x & = & 77.5(0.1954 - 0.884x + x^{2}\\x & = & 15.14 - 68.51x + 77.5x^{2}\\77.5x^{2} - 69.51x + 15.14 & = & 0\\x & = & \mathbf{0.373}\\\end{array}](/tpl/images/0136/6655/e9c11.png)


