
Chemistry, 27.07.2019 00:30 moisealafleur
For the reaction n2o4(g) m 2 no2(g), a reaction mixture at a certain temperature initially contains both n2o4 and no2 in their standard states (see the defi nition of standard state in section 6.9 ) . if kp = 0.15, which statement is true of the reaction mixture before any reaction occurs? (a) q = k; the reaction is at equilibrium. (b) q 6 k; the reaction will proceed to the right. (c) q 7 k; the reaction will proceed to the left.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
For the reaction n2o4(g) m 2 no2(g), a reaction mixture at a certain temperature initially contains...
Questions



Mathematics, 24.04.2020 20:52




Mathematics, 24.04.2020 20:52


Chemistry, 24.04.2020 20:52




Mathematics, 24.04.2020 20:53

Mathematics, 24.04.2020 20:53


Biology, 24.04.2020 20:53


Social Studies, 24.04.2020 20:53

Physics, 24.04.2020 20:53

Mathematics, 24.04.2020 20:53

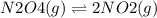
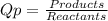
![Qp = \frac{[NO2]^{2} }{[N2O4]} \\\\Under\ standard\ state\ : \\\\Pressure\ NO2 = \ Pressure\ N2O4\ = 1\ atm\\\\Qp = \frac{[1.00]^{2} }{[1.00]} = 1](/tpl/images/0136/8396/d2f96.png)


