
Chemistry, 27.07.2019 01:10 lillianrhoades2
Menthol, the substance we can smell in mentholated cough drops, is composed of c, h, and o. a 0.1005 g sample of menthol is combusted, producing 0.2829 g of carbon dioxide and 0.1159 g of water. what is the empirical formula for menthol? if mentho has a molar mass of 156 g/mol, what is its molecular formula?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Menthol, the substance we can smell in mentholated cough drops, is composed of c, h, and o. a 0.1005...
Questions

Mathematics, 04.04.2021 06:50

Chemistry, 04.04.2021 06:50






English, 04.04.2021 06:50


Mathematics, 04.04.2021 06:50

Mathematics, 04.04.2021 06:50

Mathematics, 04.04.2021 06:50


Mathematics, 04.04.2021 06:50

Mathematics, 04.04.2021 06:50

English, 04.04.2021 06:50




Social Studies, 04.04.2021 06:50

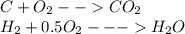
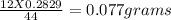 of carbon
of carbon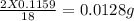 hydrogen
hydrogen

