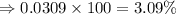Chemistry, 27.07.2019 01:20 ValeryGi3721
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopic forms of silicon. about 92.22% of silicon atoms are 27.9769amu, which have the mass of 28si; about 4.68% are 28.9764amu, which have the mass of 29si, and the remaining isotope, 30si, has a mass of 29.9737amu. calculate the percent isotopic composition of 30si."

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

You know the right answer?
"a sample of silicon has an average atomic mass of 28.084amu. in the sample, there are three isotopi...
Questions




Computers and Technology, 30.08.2021 20:50




English, 30.08.2021 20:50



Business, 30.08.2021 20:50

History, 30.08.2021 20:50

English, 30.08.2021 20:50


Social Studies, 30.08.2021 20:50

English, 30.08.2021 20:50

Mathematics, 30.08.2021 20:50



History, 30.08.2021 20:50

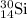 isotope is 3.09 %.
isotope is 3.09 %. .....(1)
.....(1)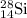 isotope be 'x'
isotope be 'x'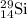 isotope:
isotope:![28.084=[(27.9769\times 0.9222)+(28.9764\times 0.0468)+(29.9737\times x)]\\\\x=0.0309](/tpl/images/0136/9885/23b7c.png)