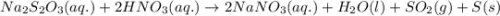Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
Na2s2o3 (aq) + 2hno3 (aq) →
a. 2nano3 (aq) + h2o (l) + so2 (g) + s (s)
b. h2o (l) + so2...
a. 2nano3 (aq) + h2o (l) + so2 (g) + s (s)
b. h2o (l) + so2...
Questions



Computers and Technology, 29.05.2020 00:57

Mathematics, 29.05.2020 00:57


Mathematics, 29.05.2020 00:57

Mathematics, 29.05.2020 00:57


Biology, 29.05.2020 00:57

English, 29.05.2020 00:57

Computers and Technology, 29.05.2020 00:57

Mathematics, 29.05.2020 00:57


Law, 29.05.2020 00:57



Mathematics, 29.05.2020 00:57


History, 29.05.2020 00:57

Mathematics, 29.05.2020 00:57