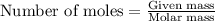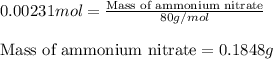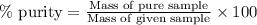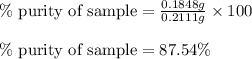Chemistry, 27.07.2019 03:10 JvGaming2001
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity can be analyzed by titrating a solution of nh4no3 with a standard naoh solution. in one experiment a 0.2111-g sample of industrially prepared nh4no3 required 22.62 ml of 0.1023 m naoh for neutralization (a) enter a net ionic equation for the reaction (include states of matter) (b) what is the percent purity of the sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1


You know the right answer?
Ammonium nitrate (nh4n03) is one of the most important nitrogen-containing fertilizers. its purity c...
Questions



Social Studies, 03.02.2021 21:20


Chemistry, 03.02.2021 21:20


Biology, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20


Biology, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20


Chemistry, 03.02.2021 21:20


Mathematics, 03.02.2021 21:20


Spanish, 03.02.2021 21:20

Social Studies, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20

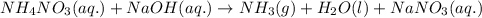
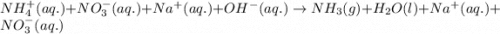
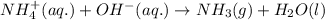
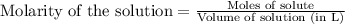
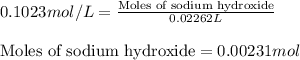
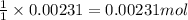 of ammonium nitrate.
of ammonium nitrate.