

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
The enthalpy change for converting 10.0 g of ice at -25.0°c to water at 80.0°c is kj. the specific...
Questions

Mathematics, 15.10.2019 06:10

History, 15.10.2019 06:10


Mathematics, 15.10.2019 06:10




History, 15.10.2019 06:10



History, 15.10.2019 06:10

Mathematics, 15.10.2019 06:10

Mathematics, 15.10.2019 06:10


Mathematics, 15.10.2019 06:10



Biology, 15.10.2019 06:10


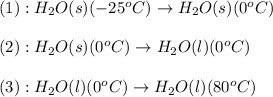
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0137/4660/5cd06.png)
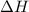 = enthalpy change = ?
= enthalpy change = ?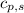 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk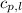 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk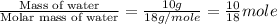
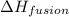 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 2.09J/gK\times (273-248)k]+\frac{10}{18}mole\times 6010J/mole+[10g\times 4.18J/gK\times (353-273)k]](/tpl/images/0137/4660/7e566.png)
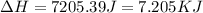 (1 KJ = 1000 J)
(1 KJ = 1000 J)

