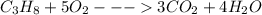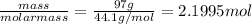He combustion of propane (c3h8) is given by the balanced chemical equation c_3h_8+5o_2\longrightarrow3co_2+4h_ 2o c 3 h 8 + 5 o 2 ⟶ 3 c o 2 + 4 h 2 o how many grams of carbon dioxide gas (co2) are produced burning 97 g of propane? round your answer to the nearest gram.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

You know the right answer?
He combustion of propane (c3h8) is given by the balanced chemical equation c_3h_8+5o_2\longrightarro...
Questions

Mathematics, 08.01.2021 18:50

Geography, 08.01.2021 18:50

Mathematics, 08.01.2021 18:50



English, 08.01.2021 18:50

Mathematics, 08.01.2021 18:50

Mathematics, 08.01.2021 18:50

English, 08.01.2021 18:50

Arts, 08.01.2021 18:50

English, 08.01.2021 18:50

Mathematics, 08.01.2021 18:50


Physics, 08.01.2021 18:50


Mathematics, 08.01.2021 18:50


Social Studies, 08.01.2021 18:50