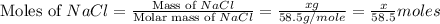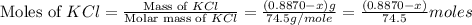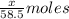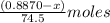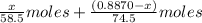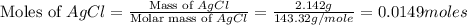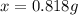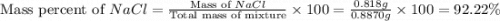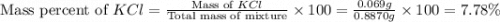Chemistry, 29.07.2019 20:20 nicolascorrea0207
A0.8870 g sample of a mixture of nacl and kcl is dissolved in water, and the solution is then treated with an excess of agno3 to yield 2.142 g of agcl. calculate the percent by mass of each compound in the mixture

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
A0.8870 g sample of a mixture of nacl and kcl is dissolved in water, and the solution is then treate...
Questions


English, 08.10.2019 18:00



Mathematics, 08.10.2019 18:00



Social Studies, 08.10.2019 18:00


English, 08.10.2019 18:00

Biology, 08.10.2019 18:00

Business, 08.10.2019 18:00


Physics, 08.10.2019 18:00

Biology, 08.10.2019 18:00

English, 08.10.2019 18:00



English, 08.10.2019 18:00

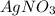 then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.
then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.