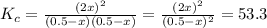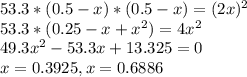Chemistry, 29.07.2019 21:10 sreeranjanig
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(=53.3 at this temperature, 0.500 mol h2 and 0.500 mol i2 were placed in a 1.00 l container to react. what concentration of hi is present at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, , for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(...
Questions


Mathematics, 19.05.2020 14:14

Chemistry, 19.05.2020 14:14

History, 19.05.2020 14:14


Social Studies, 19.05.2020 14:14

Chemistry, 19.05.2020 14:14

Chemistry, 19.05.2020 14:14


Mathematics, 19.05.2020 14:14

History, 19.05.2020 14:14






Mathematics, 19.05.2020 14:14



Mathematics, 19.05.2020 14:15

![K_{c}=\frac{[HI]^{2}}{[I_{2}][H_{2}]}](/tpl/images/0148/0680/ff3a2.png)
![[HI]={[HI]}_{0}+2x\\{[H_{2}]}={[H_{2}]}_{0}-x\\{[I_{2}]}={[I_{2}]}_{0}-x\\](/tpl/images/0148/0680/e5b4e.png)