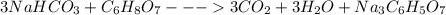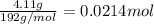Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water solution to produce a fizz as follows: 3nahco3 + c6h8o7 → 3co2 + 3h2o + na3c6h5o7 if 4.11 g of the citric acid (c6h8o7, mw = 192 g/mol) react with excess sodium bicarbonate (nahco3), how many grams of carbon dioxide (co2, mw = 44 g/mol) are formed as the solution fizzes?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1


Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water sol...
Questions


Mathematics, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30



Mathematics, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30

Spanish, 30.10.2020 22:30



Mathematics, 30.10.2020 22:30


English, 30.10.2020 22:40

Mathematics, 30.10.2020 22:40



History, 30.10.2020 22:40

Mathematics, 30.10.2020 22:40

Health, 30.10.2020 22:40