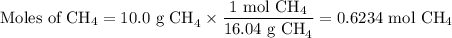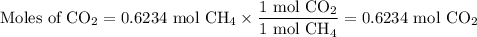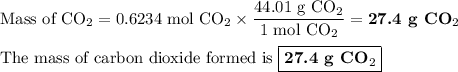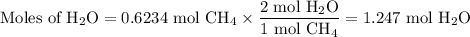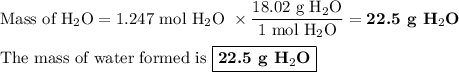Chemistry, 29.07.2019 22:20 milkshakegrande101
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left over, 27.5 grams of carbon dioxide are formed. how many grams of water are formed? ch4+ 2o2 → co2 + 2h2o

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
When 10.0 grams of ch4 reacts completely with 40.0 grams of o2 such that there are no reactants left...
Questions

Mathematics, 02.06.2021 17:40




Mathematics, 02.06.2021 17:40

Mathematics, 02.06.2021 17:40



Mathematics, 02.06.2021 17:40


Mathematics, 02.06.2021 17:40

Computers and Technology, 02.06.2021 17:40


History, 02.06.2021 17:50

Social Studies, 02.06.2021 17:50

Social Studies, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50

Arts, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50