

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
What is the ph of a solution prepared by mixing 25.00 ml of 0.10 m ch3co2h with 25.00 ml of 0.010 m...
Questions

Mathematics, 29.06.2019 13:00

Physics, 29.06.2019 13:00


Mathematics, 29.06.2019 13:00

Mathematics, 29.06.2019 13:00




English, 29.06.2019 13:00

Mathematics, 29.06.2019 13:00







History, 29.06.2019 13:00



Mathematics, 29.06.2019 13:00

![pH = pKa + log\frac{[A-]}{[HA]} ----(1)](/tpl/images/0148/5540/36d6d.png)

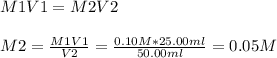
![pH = pKa + log\frac{[CH3COONa]}{[CH3COOH]} ----(1)](/tpl/images/0148/5540/1055b.png)
![pH = -logKa + log\frac{[CH3COONa]}{[CH3COOH]}](/tpl/images/0148/5540/5f73f.png)
![pH = 4.74 + log\frac{[0.005]}{[0.05]}](/tpl/images/0148/5540/63b4d.png)


