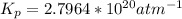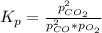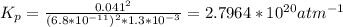Chemistry, 30.07.2019 03:20 nengliangli523
Determine the value of kp for the following reaction if the equilibrium concentrations are as follows: p(co)eq = 6.8 × 10-11 atm, p(o2)eq = 1.3 × 10-3 atm, p(co2)eq = 0.041 atm. 2 co(g) + o2(g) ⇌ 2 co2(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Determine the value of kp for the following reaction if the equilibrium concentrations are as follow...
Questions

History, 03.01.2020 19:31


Biology, 03.01.2020 19:31




History, 03.01.2020 19:31


History, 03.01.2020 19:31


Mathematics, 03.01.2020 19:31


Biology, 03.01.2020 19:31


Mathematics, 03.01.2020 19:31



Social Studies, 03.01.2020 19:31