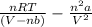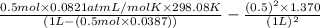Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Calculate the pressure exerted by 0.5000 mol of n2 in a 1.-l container at 25.08c a. using the ideal...
Questions

English, 30.09.2019 02:30


Health, 30.09.2019 02:30


English, 30.09.2019 02:30


Mathematics, 30.09.2019 02:30


English, 30.09.2019 02:30


English, 30.09.2019 02:30

History, 30.09.2019 02:30


English, 30.09.2019 02:30

Mathematics, 30.09.2019 02:30


History, 30.09.2019 02:30

Mathematics, 30.09.2019 02:30


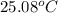 . Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.
. Or in kelvin temperature will be (25.08 + 273) K = 298.08 K.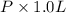 = 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]
= 0.5 mol \times 0.0821 L atm/mol K \times 298.08 K[/tex]![[P + a (\frac{n}{V})^{2}] (\frac{V}{n} - b)](/tpl/images/0149/5099/3685f.png) = RT
= RT