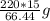Chemistry, 30.07.2019 17:10 celeste961
Achemistry student needs 15.00g of acetic acid for an experiment. he has available 220.g of a 30.2% w/w solution of acetic acid in benzene. calculate the mass of solution the student should use. if there's not enough solution, press the "no solution" button. be sure your answer has the correct number of significant digits. g ×10no solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

You know the right answer?
Achemistry student needs 15.00g of acetic acid for an experiment. he has available 220.g of a 30.2%...
Questions

Mathematics, 01.04.2020 15:52







Mathematics, 01.04.2020 15:52


Mathematics, 01.04.2020 15:52



Mathematics, 01.04.2020 15:52




Mathematics, 01.04.2020 15:53