Chemistry, 30.07.2019 22:20 palcochran1313
Given the following balanced reaction between liquid ammonia and oxygen gas to produce nitrous oxide gas and water, how many grams of water, h2o, are produced from 317 grams of ammonia and excess oxygen? (to find the molar mass in the problem use the periodic table and round the mass to the hundreds place for calculation).
(a) 224 g
(b) 335 g
(c) 503 g

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
Given the following balanced reaction between liquid ammonia and oxygen gas to produce nitrous oxide...
Questions


Biology, 05.12.2020 03:40


Mathematics, 05.12.2020 03:40

Mathematics, 05.12.2020 03:40





Mathematics, 05.12.2020 03:50





Social Studies, 05.12.2020 03:50


Mathematics, 05.12.2020 03:50

Mathematics, 05.12.2020 03:50


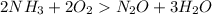
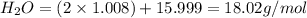
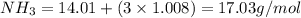
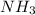 to moles
to moles 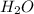 by using mole ratio of
by using mole ratio of 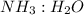 i.e., 2 : 3
i.e., 2 : 3
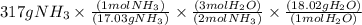
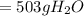 is formed.
is formed.
 is
is