Chemistry, 30.07.2019 23:20 SydneyFrank
How many moles of carbon dioxide are produced when 5.12 mol of propane gas is burned in excess oxygen? c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(l)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
How many moles of carbon dioxide are produced when 5.12 mol of propane gas is burned in excess oxyge...
Questions

English, 09.12.2021 01:00

English, 09.12.2021 01:00

Business, 09.12.2021 01:00




Chemistry, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00


English, 09.12.2021 01:00

Biology, 09.12.2021 01:00



Mathematics, 09.12.2021 01:00



Mathematics, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00

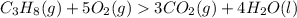
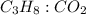 is 1 : 3
is 1 : 3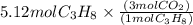
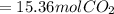 is produced.
is produced.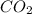 is
is