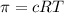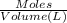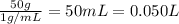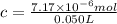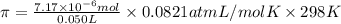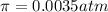Chemistry, 30.07.2019 23:30 makwoods417ow2txa
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this protein is dissolved in 50 g of water at 298 k. calculate the vapor pressure lowering, the depression in freezing point, the elevation of boiling point, and the osmotic pressure of this solution. the vapor pressure of pure water at 298 k is 23. 76 mmhg.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this...
Questions

Mathematics, 30.08.2019 09:10



Mathematics, 30.08.2019 09:10

Mathematics, 30.08.2019 09:10


History, 30.08.2019 09:10




Mathematics, 30.08.2019 09:10


Mathematics, 30.08.2019 09:10

Mathematics, 30.08.2019 09:10




Mathematics, 30.08.2019 09:10


Chemistry, 30.08.2019 09:10

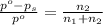
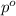 = Vapor Pressure of the pure solvent
= Vapor Pressure of the pure solvent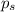 = Vapor Pressure of the solution
= Vapor Pressure of the solution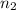 moles of solute
moles of solute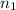 = moles of solvent
= moles of solvent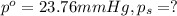
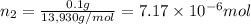
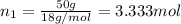
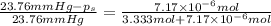
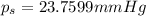
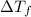 is given by:
is given by: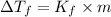
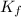 = molal depression constant of solvent
= molal depression constant of solvent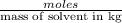
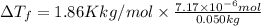
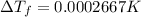
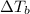 is given by:
is given by: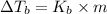
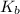 = molal elevation constant of solvent
= molal elevation constant of solvent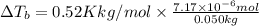
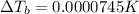
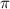 is given as:
is given as: