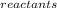Chemistry, 30.07.2019 23:40 villafana36
Calculate the entropy change for the reaction: fe2o3(s) +3c(s) -> 2fe(s) + 3co(g)
entropy data:
fe2o3(s): 90 j/k mol
c(s): 5.7 j/k mol
fe(s): 27.2 j/k mol
co(g): 198 j/k mol

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1

You know the right answer?
Calculate the entropy change for the reaction: fe2o3(s) +3c(s) -> 2fe(s) + 3co(g)
entropy...
entropy...
Questions

Business, 06.02.2021 01:00



Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00

History, 06.02.2021 01:00





Law, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00






Advanced Placement (AP), 06.02.2021 01:00

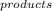 - ∑S
- ∑S