
Chemistry, 31.07.2019 04:30 marieknight689
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can form from 4 moles of hydrogen and excess oxygen. 2h2(g)+o2(g)→2h2o(l) which of the following shows calculations for a correct way to solve this problem? view available hint(s) consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can form from 4 moles of hydrogen and excess oxygen. which of the following shows calculations for a correct way to solve this problem? 4 mol h2×2 mol h2o2 mol h2=4 mol h2o 4 mol h2×2 mol h2o1 mol o2=8 mol h2o 2 mol h2×2 mol h22 mol h2o=2 mol h2o

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
How many miles of calcium oxide will be produced when 1.6 miles of iron (iii) oxide react with calcium phosphate
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions

Mathematics, 23.08.2019 11:20

Biology, 23.08.2019 11:20

Mathematics, 23.08.2019 11:20


History, 23.08.2019 11:20



History, 23.08.2019 11:20

English, 23.08.2019 11:20


Mathematics, 23.08.2019 11:30




Mathematics, 23.08.2019 11:30

Chemistry, 23.08.2019 11:30

Health, 23.08.2019 11:30



Mathematics, 23.08.2019 11:30

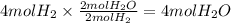
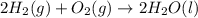
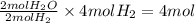 of water.
of water.

