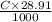Chemistry, 31.07.2019 17:20 drealtania21
Enter your answer in the provided box. sodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. a sodium hydroxide solution was standardized by titrating 28.58 ml of 0.1851 m standard hydrochloric acid. the initial buret reading of the sodium hydroxide was 1.98 ml, and the final reading was 30.89 ml. what was the molarity of the base solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Enter your answer in the provided box. sodium hydroxide is used extensively in acid-base titrations...
Questions

Chemistry, 01.09.2019 11:10




Mathematics, 01.09.2019 11:10


Mathematics, 01.09.2019 11:10

Biology, 01.09.2019 11:10


Social Studies, 01.09.2019 11:10

History, 01.09.2019 11:10



History, 01.09.2019 11:10


Mathematics, 01.09.2019 11:10


English, 01.09.2019 11:10


English, 01.09.2019 11:10


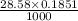 moles
moles