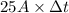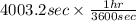Chemistry, 31.07.2019 18:20 chanel2371
Chromium plating can be applied by electrolysis to objects according to the following unbalanced half-reaction: cr2o72- + e- + h+→ cr(s) + h2ohow long (in hours) would it take to apply a chromium plating 0.010 mm thick to a car bumper with a surface area of 0.25 m2 in a cell with a current of 25.0 a? the density of chromium is 7.19 g/cm3.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Chromium plating can be applied by electrolysis to objects according to the following unbalanced hal...
Questions

Mathematics, 16.10.2020 14:01

Biology, 16.10.2020 14:01

Geography, 16.10.2020 14:01



Computers and Technology, 16.10.2020 14:01



Mathematics, 16.10.2020 14:01

Physics, 16.10.2020 14:01


Biology, 16.10.2020 14:01

Social Studies, 16.10.2020 14:01

Computers and Technology, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01



Social Studies, 16.10.2020 14:01


History, 16.10.2020 14:01

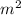 , current (I) = 25 A
, current (I) = 25 A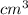
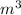 =
= 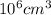
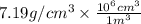
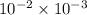 =
= 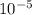 m.
m.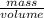
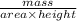
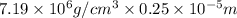
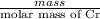
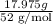
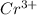 is 579000 C
is 579000 C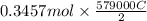 = 100080 C.
= 100080 C. 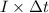
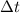 = time in seconds
= time in seconds