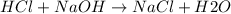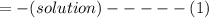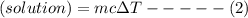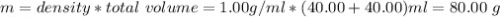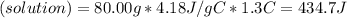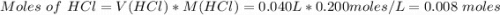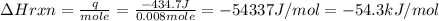When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee-cup calorimeter, the temperature of the resulting solution rises to 22.8°c. assume that the volumes are additive, the specific heat of the solution is 4.18 jg -1°c -1 and that the density of the solution is 1.00 g ml -1 calculate the enthalpy change, δh in kj for the reaction:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1


You know the right answer?
When 40.0 ml of 0.200 m hcl at 21.5°c is added to 40.0 ml of 0.200 m naoh also at 21.5°c in a coffee...
Questions



Physics, 05.05.2021 01:00


Computers and Technology, 05.05.2021 01:00


History, 05.05.2021 01:00

English, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00


Chemistry, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00