Chemistry, 31.07.2019 18:30 bixbylily95
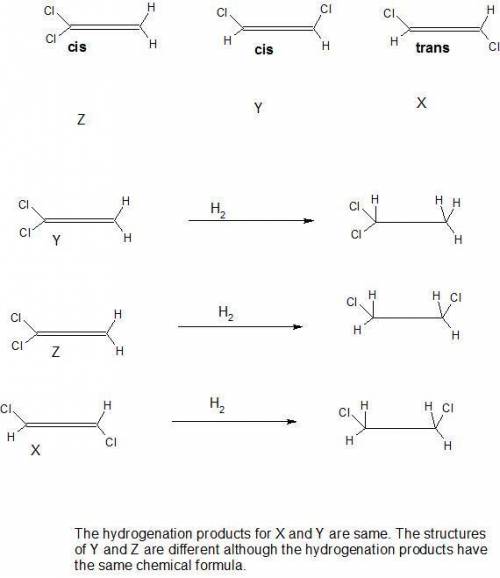
Be sure to answer all parts. there are three different dichloroethylenes (molecular formula c2h2cl2), which we can designate x, y, and z. compound x has no dipole moment, but compound z does. compounds x and z each combine with hydrogen to give the same product: c2h2cl2(x or z) + h2 → clch2―ch2cl what are the structures of x, y, and z? be sure to include lone pair electrons

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Be sure to answer all parts. there are three different dichloroethylenes (molecular formula c2h2cl2)...
Questions


Mathematics, 31.10.2021 02:40

Mathematics, 31.10.2021 02:40




Mathematics, 31.10.2021 02:40

Mathematics, 31.10.2021 02:40

Mathematics, 31.10.2021 02:40


Mathematics, 31.10.2021 02:40

Social Studies, 31.10.2021 02:40



Mathematics, 31.10.2021 02:40


Chemistry, 31.10.2021 02:40