

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [cl2]. if the...
Questions

Mathematics, 15.06.2020 13:57

Mathematics, 15.06.2020 13:57







English, 15.06.2020 13:57

Mathematics, 15.06.2020 13:57


Mathematics, 15.06.2020 13:57

Spanish, 15.06.2020 13:57



Spanish, 15.06.2020 13:57




Spanish, 15.06.2020 13:57

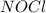 is,
is, 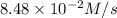
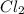 =
= 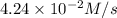
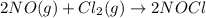
![\text{Rate of disappearance}=-\frac{1}{2}\frac{d[NO]}{dt}=-\frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/aee73.png)
![\text{Rate of formation}=\frac{1}{2}\frac{d[NOCl]}{dt}](/tpl/images/0155/2847/25db6.png)
![\frac{1}{2}\frac{d[NOCl]}{dt}=-\frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/f6a9b.png)
![\frac{d[NOCl]}{dt}=2\times \frac{d[Cl_2]}{dt}](/tpl/images/0155/2847/71d98.png)
![\frac{d[NOCl]}{dt}=2\times (4.24\times 10^{-2}M/s)=8.48\times 10^{-2}M/s](/tpl/images/0155/2847/fdae3.png)


