
Chemistry, 31.07.2019 18:30 shortyyashaun
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is the concentration of the hcl solution? (3 points)
balanced equation: naoh + hcl yields nacl + h2o
0.36 m hcl
0.70 m hcl
1.1 m hcl
1.4 m hcl

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2

Chemistry, 23.06.2019 12:00
Asubstance that will change shape to fit its container but has a definite volume is in a phase of matter
Answers: 1
You know the right answer?
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is th...
Questions





Mathematics, 20.10.2019 03:00

History, 20.10.2019 03:00

Mathematics, 20.10.2019 03:00




English, 20.10.2019 03:00



Physics, 20.10.2019 03:00

Chemistry, 20.10.2019 03:00

Computers and Technology, 20.10.2019 03:00

History, 20.10.2019 03:00

Mathematics, 20.10.2019 03:00

Mathematics, 20.10.2019 03:00


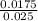 = 0.7M
= 0.7M

