
Chemistry, 31.07.2019 19:10 brianlykid3042
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 ⋅ 10-4. calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid. the ka of formic acid is 1.77 10-4. 0.859 0.0180 3.79 2.25 ⋅ 10-5 6.94

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Calculate the percent ionization of formic acid (hco2h) in a solution that is 0.125 m in formic acid...
Questions




English, 06.04.2020 21:51

Mathematics, 06.04.2020 21:52


English, 06.04.2020 21:52

Spanish, 06.04.2020 21:52

Mathematics, 06.04.2020 21:52


English, 06.04.2020 21:52

History, 06.04.2020 21:52



Physics, 06.04.2020 21:52

Mathematics, 06.04.2020 21:52


Advanced Placement (AP), 06.04.2020 21:52

Advanced Placement (AP), 06.04.2020 21:52

History, 06.04.2020 21:52

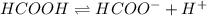
![\frac{[HCOO^{-}][H^{+}]}{[HCOOH]}=K_{a}(HCOOCH)](/tpl/images/0155/4252/45db7.png)
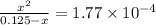
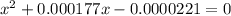
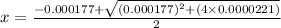
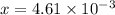 M
M![[H^{+}]=4.61\times 10^{-3}M](/tpl/images/0155/4252/38c16.png)
![\frac{[H^{+}]}{initial concentration of HCOOH}\times 100](/tpl/images/0155/4252/17262.png) =
= 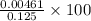 = 3.69%
= 3.69%

