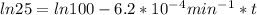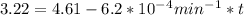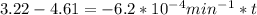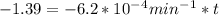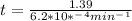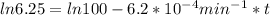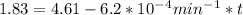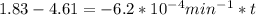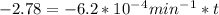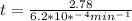Chemistry, 31.07.2019 20:30 YODIIZ6590
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the rate law is first order in n2o5.) how long would it take for the concen- tration of n2o5 to decrease to 25% of its initial value? to 6.25% of its initial value?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the r...
Questions



Mathematics, 25.10.2021 14:00




Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00


English, 25.10.2021 14:00


French, 25.10.2021 14:00




Biology, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00



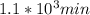 ,
, 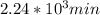 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and 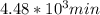 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% . 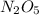 . For first order reaction, rate constant and half life are related to each other as:
. For first order reaction, rate constant and half life are related to each other as: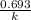
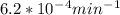
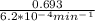
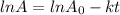
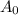 is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.