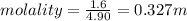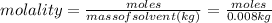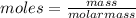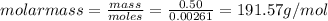Chemistry, 31.07.2019 20:40 linshweyioo5442
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0 g of benzene. the solution freezes at 3.9°c. can the chemist conclude that the compound is cocaine (c17h21n04)? what assumptions are made in the analysis? the freezing point of benzene is 5.5°c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0...
Questions

Mathematics, 23.09.2021 15:50

Medicine, 23.09.2021 15:50


Mathematics, 23.09.2021 15:50

Biology, 23.09.2021 15:50

Mathematics, 23.09.2021 15:50

Mathematics, 23.09.2021 15:50


Mathematics, 23.09.2021 15:50


History, 23.09.2021 15:50

Mathematics, 23.09.2021 15:50



History, 23.09.2021 15:50

English, 23.09.2021 15:50

Mathematics, 23.09.2021 15:50


Engineering, 23.09.2021 15:50