
Chemistry, 31.07.2019 21:20 smelcher3900
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l) + 1/2 o2(g)at 20.0 °c, the half-life for the reaction is 3.92 x 104 seconds. if the initial concentration of hydrogen peroxide is 0.52 m, what is the concentration after 7.00 days? 1.2 x 10-5 m0.034 m0.074 m0.22 m0.52 m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l)...
Questions




















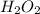 after 7.00 days is
after 7.00 days is 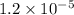 M
M![[H_{2}O_{2}]=[H_{2}O_{2}]_{0}\times (0.5)^{(\frac{t}{t_{0.5}})}](/tpl/images/0155/7555/57b7f.png)
![[H_{2}O_{2}]_{0}](/tpl/images/0155/7555/0e2b6.png) is initial concentration of
is initial concentration of 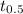 is half-life
is half-life![[H_{2}O_{2}]= 0.52\times (0.5)^{\frac{604800}{39200}}](/tpl/images/0155/7555/78886.png)
![[H_{2}O_{2}]](/tpl/images/0155/7555/955b6.png) =
= 


