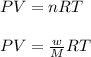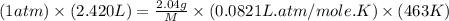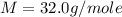Chemistry, 31.07.2019 21:20 rashawng2005
Asample of an unknown compound is vaporized at 190.°c. the gas produced has a volume of 2420.ml at a pressure of 1.00 atm, and it weighs 2.04 g. assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
Asample of an unknown compound is vaporized at 190.°c. the gas produced has a volume of 2420.ml at a...
Questions


Mathematics, 20.10.2019 03:30





English, 20.10.2019 03:30


Advanced Placement (AP), 20.10.2019 03:30








Chemistry, 20.10.2019 03:30

Mathematics, 20.10.2019 03:30

Mathematics, 20.10.2019 03:30