Chemistry, 31.07.2019 22:10 richdakid26
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is the concentration of the hcl solution? (3 points)
balanced equation: naoh + hcl yields nacl + h2o
select one:
a. 0.36 m hcl
b. 0.70 m hcl
c. 1.1 m hcl
d. 1.4 m hcl

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is th...
Questions




















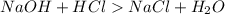
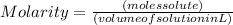 and its unit is mol/L
and its unit is mol/L