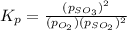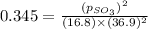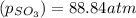Chemistry, 31.07.2019 23:10 yournerdybirdyp43oi3
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3 (g) at equilibrium, the partial pressure of so2 is 36.9 atm and that of o2 is 16.8 atm. the partial pressure of so3 is atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3...
Questions



History, 31.08.2019 14:20





Mathematics, 31.08.2019 14:20


Mathematics, 31.08.2019 14:20


Mathematics, 31.08.2019 14:20


Mathematics, 31.08.2019 14:20

Computers and Technology, 31.08.2019 14:20

Mathematics, 31.08.2019 14:20



History, 31.08.2019 14:20

Biology, 31.08.2019 14:20

 is, 88.84 atm
is, 88.84 atm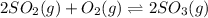
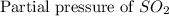 = 36.9 atm
= 36.9 atm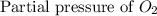 = 16.8 atm
= 16.8 atm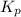 will be,
will be,