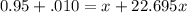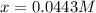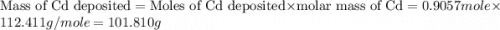Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead” (at 298 k) assuming that the initial mass of both metal electrodes are 100 g and all solutions in the cell are exactly 1.0 l. fe | fe2+ (0.10 m) || cd2+ (0.95 m) | cd

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

You know the right answer?
Determine the mass of the metal at the cathode of the following galvanic cell when the cell is “dead...
Questions



Social Studies, 27.10.2019 10:43

Mathematics, 27.10.2019 10:43


English, 27.10.2019 10:43


Social Studies, 27.10.2019 10:43

Mathematics, 27.10.2019 10:43

Physics, 27.10.2019 10:43


Biology, 27.10.2019 10:43



History, 27.10.2019 10:43

Business, 27.10.2019 10:43

Mathematics, 27.10.2019 10:43

Mathematics, 27.10.2019 10:43


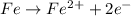
![E^0_{[Fe^{2+}/Fe]}=-0.44V](/tpl/images/0156/0778/59478.png)
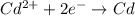
![E^0_{[Cd^{2+}/Cd]}=-0.40V](/tpl/images/0156/0778/d77be.png)
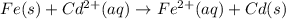
![E^0=E^0_{[Cd^{2+}/Cd]}-E^0_{[Fe^{2+}/Fe]}](/tpl/images/0156/0778/49ec3.png)
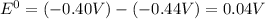
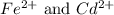 .
.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/33164.png)
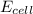 = emf of the cell = 0 (for dead cell)
= emf of the cell = 0 (for dead cell)![0=0.04-\frac{0.0592}{2}\log \frac{[Fe^{2+}]}{[Cd^{2+}]}](/tpl/images/0156/0778/3fae7.png)
![\frac{[Fe^{2+}]}{[Cd^{2+}]}=22.695](/tpl/images/0156/0778/c0251.png)
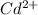 be, 'x'.
be, 'x'.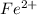 will be,
will be, ![22.695\times [Cd^{2+}]=22.695x](/tpl/images/0156/0778/44a3e.png)
![[Cd^{2+}]+[Fe^{2+}]=x+22.695x](/tpl/images/0156/0778/08dba.png)