
Chemistry, 01.08.2019 00:20 bcozort611
For the reaction co(g) + 3 h2(g) ⇌ h2o(g) + ch4(g), kc = 189 at 1000 k. if a vessel is filled with these gases such that the initial concentrations are [co] = 0.0360 m, [h2] = 0.0450 m, [h2o] = 0.0200 m, and [ch4] = 0.0310 m, in which direction will a reaction occur and why?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
For the reaction co(g) + 3 h2(g) ⇌ h2o(g) + ch4(g), kc = 189 at 1000 k. if a vessel is filled with t...
Questions

History, 22.10.2019 23:00



Mathematics, 22.10.2019 23:00

Biology, 22.10.2019 23:00



Mathematics, 22.10.2019 23:00

Mathematics, 22.10.2019 23:00




Mathematics, 22.10.2019 23:00

Mathematics, 22.10.2019 23:00

Biology, 22.10.2019 23:00

Chemistry, 22.10.2019 23:00




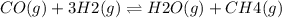
![Qc = \frac{[Products]}{[Reactants} \\\\For\ the\ given\ reaction\\\\Qc = \frac{[H2O][CH4]}{[CO][H2]^{3} } -----(1)](/tpl/images/0156/2334/36d57.png)
![Qc= \frac{[0.0200][0.0310]}{[0.0360][0.0450]^{3} } =189](/tpl/images/0156/2334/3395f.png)


