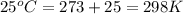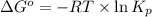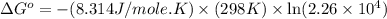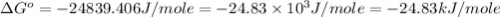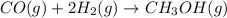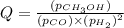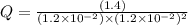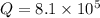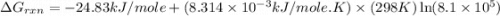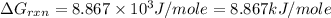Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Consider the following reaction: co(g)+2h2(g)⇌ch3oh(g) kp=2.26×104 at 25 ∘c. calculate δgrxn for th...
Questions













Computers and Technology, 26.08.2019 17:10






Computers and Technology, 26.08.2019 17:10

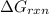 is,
is, 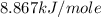
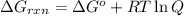 ............(1)
............(1)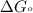 = standard Gibbs free energy
= standard Gibbs free energy