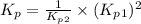Chemistry, 01.08.2019 01:20 christophergaudette0
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)kp=5.3 2no(g)⇌n2(g)+o2(g)kp=2.1×1030 use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: n2(g)+o2(g)+br2(g)⇌2nobr(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)...
Questions




Mathematics, 21.05.2021 17:50

Chemistry, 21.05.2021 17:50


English, 21.05.2021 17:50

Mathematics, 21.05.2021 17:50

Mathematics, 21.05.2021 17:50

Mathematics, 21.05.2021 17:50

History, 21.05.2021 17:50

Mathematics, 21.05.2021 17:50

Arts, 21.05.2021 17:50

Physics, 21.05.2021 17:50



Biology, 21.05.2021 17:50



Mathematics, 21.05.2021 17:50


 ;
; 
 ;
; 
 ;
;