
Chemistry, 01.08.2019 01:30 ilianysacosta10
Ethanol (c 2h 6o(l), δh o f = -277.69 kj/mol) can be made by reaction of ethylene (c 2h 4(g) δh o f = 52.26 kj/mol) with water (δh o f = -285.83 kj/mol). what is the enthalpy of reaction for this process?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Ethanol (c 2h 6o(l), δh o f = -277.69 kj/mol) can be made by reaction of ethylene (c 2h 4(g) δh o f...
Questions

Medicine, 26.05.2021 20:30

Mathematics, 26.05.2021 20:30

Mathematics, 26.05.2021 20:30

Mathematics, 26.05.2021 20:30


Mathematics, 26.05.2021 20:30


Mathematics, 26.05.2021 20:30



History, 26.05.2021 20:30

Mathematics, 26.05.2021 20:30




Mathematics, 26.05.2021 20:30


Physics, 26.05.2021 20:30

Mathematics, 26.05.2021 20:30

Spanish, 26.05.2021 20:30

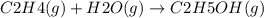
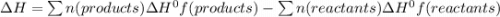
![\Delta H = 1\Delta H^{0}f(C2H6O)-[1\Delta H^{0}f(C2H4)+1\Delta H^{0}f(H2O)]](/tpl/images/0156/4062/38e6e.png)


