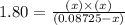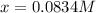Chemistry, 01.08.2019 02:20 tinapersaud1587
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80 at 250 ∘c a 0.349 mol sample of pcl5(g) is injected into an empty 4.00 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(=1.80...
Questions


History, 16.04.2020 19:27



Mathematics, 16.04.2020 19:27




Mathematics, 16.04.2020 19:27

Mathematics, 16.04.2020 19:27






Computers and Technology, 16.04.2020 19:28



Social Studies, 16.04.2020 19:28

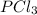 and
and 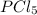 at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M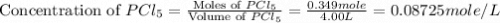
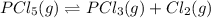
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0156/5529/73fe0.png)