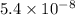Chemistry, 01.08.2019 02:20 kdenormandie3122
In air, at 25 ºc and 1.00 atm, the concentrations of n2 and o2 are 0.033 m and 0.00180, respectively. the reaction n2(g) + o2 (g) 2 no (g) has kc= 4.8 x 10-11 at 25 °c taking the given concentrations as the initial concentrations, calculate the equilibrium concentration of no at 25 °c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
In air, at 25 ºc and 1.00 atm, the concentrations of n2 and o2 are 0.033 m and 0.00180, respectively...
Questions

English, 18.10.2020 01:01






Biology, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01


Social Studies, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

Advanced Placement (AP), 18.10.2020 01:01

Business, 18.10.2020 01:01


Arts, 18.10.2020 01:01

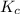 is
is 
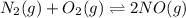
![K_{c} = \frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0156/5544/a7a07.png)
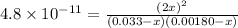
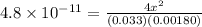
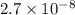 M
M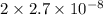 M =
M =