
Chemistry, 01.08.2019 03:10 dwilburn01
For a particular redox reaction, no is oxidized to no−3 and ag+ is reduced to ag . complete and balance the equation for this reaction in basic solution. the phases are optional. balanced reaction: no + ag^{+} -> no_{3}^{-} + ag no+ag+⟶no−3+ag

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3

You know the right answer?
For a particular redox reaction, no is oxidized to no−3 and ag+ is reduced to ag . complete and bala...
Questions



Computers and Technology, 28.07.2019 04:34


Biology, 28.07.2019 04:34


Biology, 28.07.2019 04:34

History, 28.07.2019 04:34



English, 28.07.2019 04:34

Biology, 28.07.2019 04:34


Mathematics, 28.07.2019 04:34

Spanish, 28.07.2019 04:34


English, 28.07.2019 04:34

Physics, 28.07.2019 04:34

History, 28.07.2019 04:34

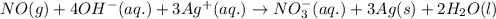
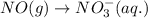
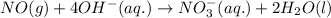
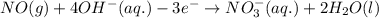 ................(1)
................(1)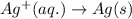
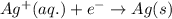 ...........(2)
...........(2) equation (2):
equation (2):

