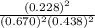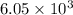Chemistry, 01.08.2019 03:20 makeithappen60
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 bar, 0.438 bar, and 0.228 bar, respectively. calculate the new equilibrium pressures if the volume of the reaction vessel is doubled.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

You know the right answer?
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 ba...
Questions

English, 28.10.2020 01:00


Biology, 28.10.2020 01:00

English, 28.10.2020 01:00


History, 28.10.2020 01:00

Mathematics, 28.10.2020 01:00


Spanish, 28.10.2020 01:00


Mathematics, 28.10.2020 01:00



Health, 28.10.2020 01:00



Mathematics, 28.10.2020 01:00



Mathematics, 28.10.2020 01:00

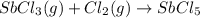
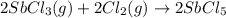
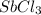 is 0.670 bar,
is 0.670 bar, 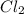 is 0.438 bar and
is 0.438 bar and 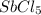 is 0.228 bar.
is 0.228 bar. ![K_{p} = \frac{[P_{SbCl_{5}}]^{2}}{[P_{SbCl_{3}}]^{2}[P_{Cl_{2}}]^{2}}](/tpl/images/0156/7126/bcfbb.png)